MEDICAL DEVICE ANALYSIS
Introduction
Regulatory Information
The market of medical devices is highly regulated. In the European Union (EU), the Medical Device Regulation (MDR) is applied, the US devices are regulated by the Food and Drug Administration (FDA). In this regard, both MDR and FDA define its own requirements for medical devices and associated testing to which manufacturers must comply with.
According to the MDR, manufacturers are obligated to systematically evaluate and record the risk emerged for medical devices. In this context, general safety and risk aspects can be managed by application of ISO 14971 and ISO 10993. Herein, the ISO 10993 series of standards focuses on biocompatibility for biological and chemical properties and patient safety of the considered devices. Thus, for device manufacturers to understand the chemical properties in order to assess risk, BV-Schwerin provides an outstanding analytical service using the latest techniques.
 Techniques
Techniques
Sample Preparation
|
Newly opened Laboratory for medical devices in 2023. Just with the latests equipments, such as: • Orbital Shaker |
Chemical Characterisation
|
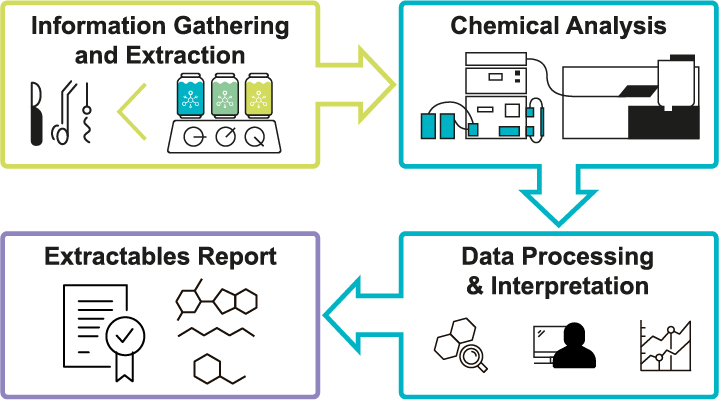
Extractables and Leachables with state of the art analytical methods |
|
Gas Chromatography (GC) |
Liquid Chromatography (LC) |
Inductively Coupled Plasma (ICP) |
Medical Certified
|
DAkkS ISO 17025 accredited |
SCOPE
ISO 10993 Screening Analysis
The combination of our state-of-the-art equipment and a highly skilled team enables BV-Schwerin to provide identification and quantification for an unexpectedly high number of compounds. Furthermore, our precise analyses reduce your uncertainty to a minimum, even for unknown substances. Thus, with BV-Schwerin, you will be on the best path when evaluating risks of your medical devices.
CMR-Related Substance List
CMR substances are chemicals that are carcinogenic, mutagenic or toxic to reproduction. They are grouped into different risk classes. Class Ia and Ib are the highest risk classes and are therefore mentioned separately in the MDR. The use of such substances must always be avoided and therefore requires targeted monitoring.
BV has developed a method to specifically search for these substances. The innovative approach at BV-Schwerin is based on a rapid screening method that first searches qualitatively for the substances. In case of findings, a quantification is carried out using confirmed analytical methods, so that an exact concentration can be determined.
As a result, you benefit from a complete monitoring of your products starting already in the development stage. If such substances are already identified in components before they are integrated into the medical device, resources - especially time and costs - can be saved.
If you would like to learn more about this complex and highly innovative method, please contact us!
Contact
|
Andreas Dumrath |
Alexander Laubach |
|
Regulatory Affairs |
Laboratory Expert |
||
|
+ 49 40 74041 1234 |
+ 49 40 74041 0047 |
||
|
andreas.dumrath@bureauveritas.com |
+ 49 174 1753086 |
||
|
alexander.laubach@bureauveritas.com |